Tools Test Drive: LiquiBand Exceed Topical Skin Adhesive
Dr. James Greenberg gives his review of the LiquiBand Exceed Topical Skin Adhesive.

Background
As a proud wound closure materials geek, I view 3 patents as the most significant modern inventions in the field: US patent 3,297,0331 for polyglycolic acid suture (1967), US patent 8,721,681 B22 for barbed suture (2014) and US patent 5,530,0373 for sterilizing cyanoacrylate (1996). The last of the triad essentially enabled Krazy® Glue (Elmer’s Products, Westerville, OH) to become Dermabond® (Ethicon, Inc., Somerville, NJ) and changed the way millions of wounds have been and will be reapproximated.
Design/Functionality
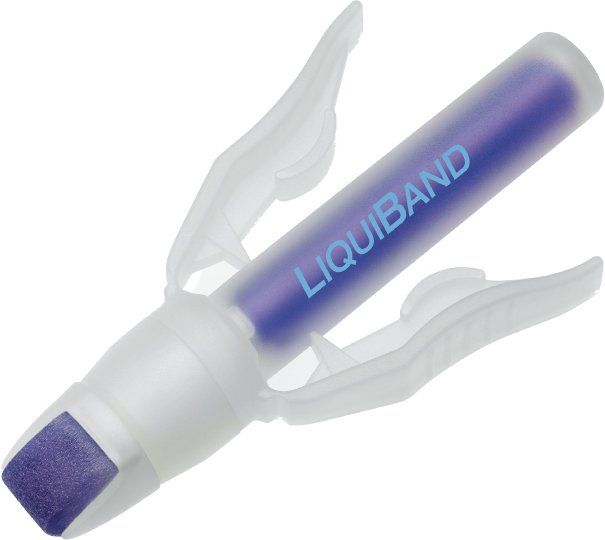
LiquiBand ExceedTM Topical Skin Adhesive is sterile 2-octyl cyanoacrylate contained in a 0.8-g ampoule encased in a proprietary dispenser for single use. While each manufacturer of a 2-octyl cyanoacrylate topical skin adhesive claims their version is the best due to special additives and processes that alter the viscosity, as a user, the biggest differences seem to be in the dispensers. With its unique felt tip and pressure-producing wings, the LiquiBand ExceedTM dispenser was by far the best topical skin adhesive dispenser I have ever used. It consistently produced an even distribution of adhesive over wounds without clogging, enabling me to use all 0.8 g of glue.
Innovation
Advanced Medical Solutions, Ltd., neither invented cyanoacrylate nor figured out how to sterilize it so I can’t give them too many cleverness points. However, their felt-tipped, winged dispenser is a big improvement over the competition so kudos to them.
Value
2-octyl cyanoacrylate is essentially a commodity product that will usually be subject to bundled pricing and thus highly variable in its cost. From my perspective, the real value discriminator between products is the dispensers. With some dispensers, the tips clog before all of the adhesive is delivered, often necessitating opening a second applicator and doubling the cost. With the LiquiBand ExceedTM dispenser I was able to use all of the adhesive in the ampoule every time. THAT…is good value.
Summary
This is simple. If you use topical skin adhesives for wound closure, you should try LiquiBand ExceedTM to see for yourself and your institution whether this is a good alternative to what you are currently using.
The views of the author are personal opinions and do not necessarily represent the views of Contemporary OB/GYN.
References:
- Schmitt EE, inventor; American Cyanamid Company, assignee. Surgical Sutures. US patent 3,297,033. January 10, 1967.
- Ruff, GL, inventor; Ethicon, Inc. assignee. Barbed suture in combination with surgical needle. US patent 8,721,681 B2. May 13, 2014.
- McDonnell PF, inventor; Loctite, Ltd, assignee. Sterilized cyanoacrylate adhesive composition, and a method of making such a composition. US patent 5,530,037. June 25, 1996.
Buprenorphine use in pregnancy linked to decreased fetal breathing movements
May 18th 2024According to a poster presented at ACOG 2024, use of the synthetic opioid buprenorphine depressed fetal breathing in biophysical profile assessments, but had no significant impact on other factors like amniotic fluid index or fetal tone.
Read More
Laparoscopic RFA linked to enhanced pregnancy outcomes in uterine fibroid patients
May 18th 2024A recent study presented at the 2024 ACOG Clinical and Scientific Meeting reveals that laparoscopic radiofrequency ablation significantly improves pregnancy outcomes for women with uterine leiomyomas.
Read More
Identifying gaps in syphilis treatment and prenatal care among pregnant individuals
May 17th 2024Preventing congenital syphilis comes down to quick diagnosis and treatment of the infection in pregnancy, and the number of missed opportunities to do so in the United States continues to grow.
Read More

